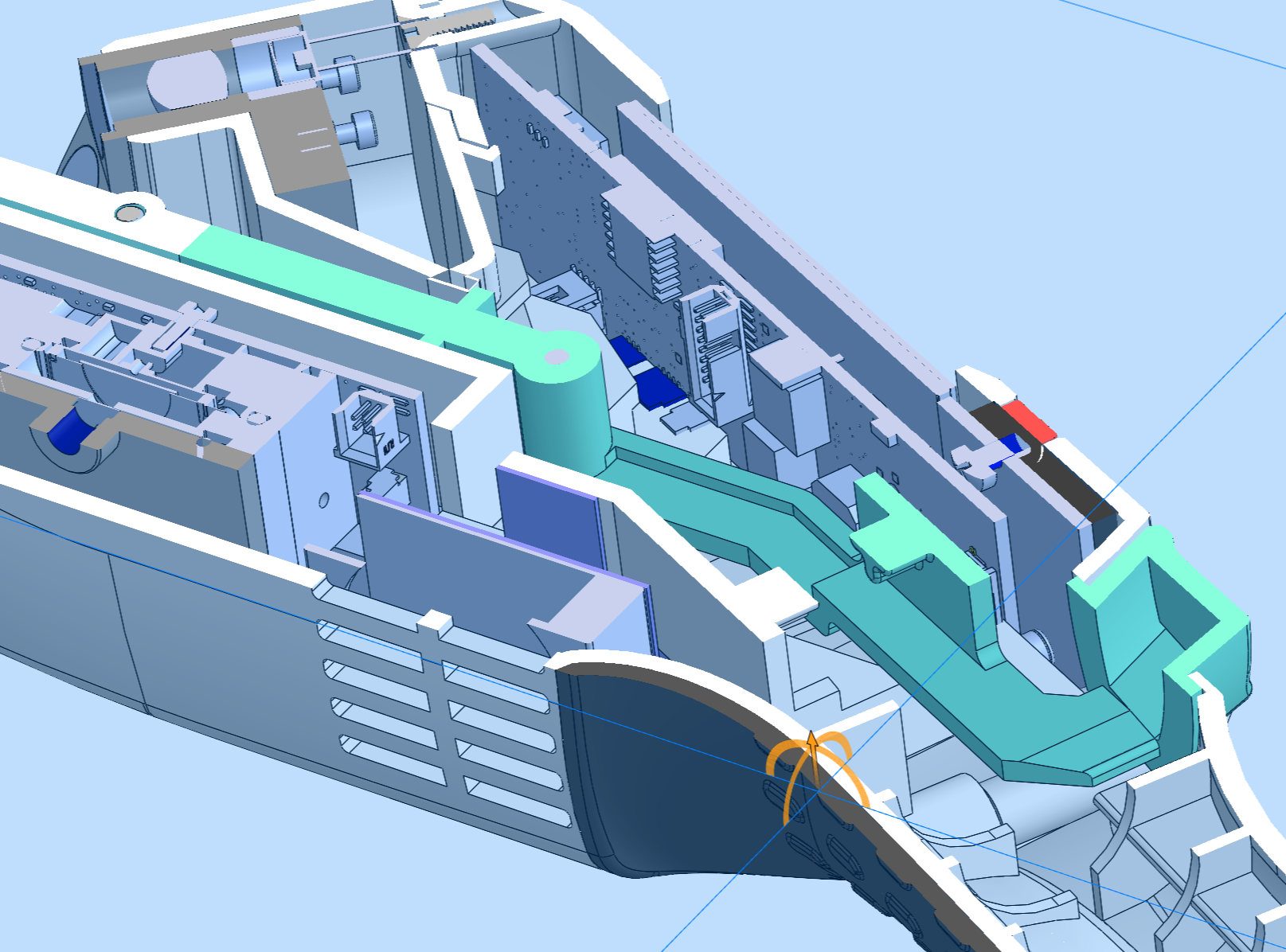
Medical Device Design Company
Scientific / Laboratory
We specialize in developing instruments for in-field use or in a protected, controlled environment. Every environment has its own unique challenges.
We partner with companies to create devices so their technicians and scientists can do their best work.
Our Unique Benefits
Decades of product development experience have given us valuable insights into what works and what doesn’t work. We understand that no two projects are alike, and we've discovered unique benefits that provide high-value results for you.
Adaptive Approach
Successful projects are rarely straightforward, which is why we have implemented an adaptive approach to product development, ensuring that we remain agile and responsive to change or new information. We pride ourselves on being flexible and adaptable to your needs, providing a development process that can adjust to important data and feedback gathered beyond the initial project brief. This adaptability proves invaluable in identifying unforeseen risks that might otherwise jeopardize your budgets and schedules.
Implied Engineering
Our high-value method of CAD implementation. There is often a gap between “design” CAD and “engineering” CAD. Some projects don’t require a full-blown mechanical engineering effort but still need design solutions that are manufacturing-optimized. We offer the flexibility to assess whether our implied engineering level of CAD design aligns with the specific needs of your project or particular phases within the development process. Additionally, we are equipped to implement engineering CAD and Design for Manufacturability (DFM) levels of CAD as needed.
INDUSTRIAL DESIGN
The scientific/laboratory sector requires a focus on developing products that perfectly blend functionality, aesthetics, innovation, and manufacturability. We integrate art with science, capturing your technology and creating a solution that will seamlessly function within laboratory settings or specific environments where tests/analyses will be performed using diagnostic devices. With a primary focus on user-centric design, our industrial design process not only understands the need and importance of designing for the user but also ensures that these solutions result in business success through meaningful ROI.
Our adaptive process allows for necessary proof-of-concept modeling and discussions to take place, with the goal of either eliminating unnecessary elements or confirming required functionality. As a medical device design company, we guide your product's transition from early concept development into manufacturing, optimizing the process to ensure design integrity is maintained throughout the development of scientific analyzers and medical devices aiding in drug delivery systems.
ENGINEERING
Our designers combine Industrial Design and Mechanical Engineering to create manufacturing-optimized designs for scientific devices and laboratory equipment. These efforts involve rapid prototyping as a method to gain an in-depth understanding of a design solution and further understand the technical limitations. Applying scientific principles and mathematical concepts to design solutions helps validate market readiness through collaborative team brainstorming efforts. This process allows for creative design thinking while addressing technical constraints, resulting in successful project outcomes.
Efficient workflow with engineering teams and relationships with global suppliers provide seamless continuity in DFM and component sourcing. Our team follows an iterative process - solving complex design and engineering challenges by repeating each step as many times as needed to provide a confident, seamless product solution.
PRODUCT VISUALIZATION
Visually appealing representations of products makes it easier for people to make informed decisions and appreciate the design, features, and functionality of a product. It is a helpful stage in the development process to make better decisions about design details and directions before investing more substantial capital to bring your product into the scientific and laboratory market. This critical tool is an important communication effort between designers, manufacturers, and business stakeholders. Evaluation efforts often rely solely on product imagery in 2D or 3D, for internal design and development purposes.
DESIGN STRATEGY & RESEARCH
An effective design strategy is critical to set the overall direction, goals, and methods for the ensuing design process. The research component provides empirical data and insights that guide and validate design decisions. The synergy between the two ensures that design solutions are not only aesthetically pleasing, but also effective, user-focused, and aligned with broader business objectives.
To understand the preferences and psychology of a brand’s audience, research helps to identify focused data points. Strong design strategy and research implementation will typically provide a smoother and quicker development process.
PROTOTYPING
Medical devices, scientific devices, and laboratory equipment require numerous iterations of physical models and prototypes to dial in optimal ergonomics and functionality. One of the most critical phases in the product development process, prototypes provide tangible objects to evaluate and test. Whether it’s an appearance prototype, functional prototype, or proof-of-concept model, your product solution must be evaluated at various levels before moving forward with full-scale production. The earlier prototypes can be built and tested, the sooner decisions can be made to validate processes such as appearance, functionality, assembly, and servicing.
The adaptive process we implement for product development will occasionally require unforeseen prototyping efforts - typically smaller proof-of-concept models that are built to confirm a specific function, ergonomic balance, or visual validation. Careful consideration is taken to understand the use scenario for lab settings and testing locations - optimizing details and efficiency.
MANUFACTURING SUPPORT
Whether we’re collaborating with your manufacturing resources or ours, we provide insight into many potential risks that may arise - supply chain interruptions, EOL components, manufacturing delays due to scope creep, and schedule slips. Through our contract partners, we can help identify new opportunities to ensure a smooth manufacturing process such as first article part inspections, internal or external testing and reporting, pilot runs, and initial production units. An efficient and effective production strategy for your product is critical.
BRAND DEVELOPMENT
A strong and positive brand perception that is differentiated from competitors is a core building block for the scientific and laboratory sector. The brand is the soul of a company. Leveraging our expertise as a Medical Device Design Company, we offer comprehensive support in building and managing your brand to give product solutions a unique and recognizable presence in a laboratory setting or specific environments where tests/analysis will be performed. Powerful branding is built on the aesthetic features of design elements as well as its messaging and emotional appeal.
Feedback provided by user research not only contributes to a better-targeted and financially successful brand but also proves essential in diverse fields, from medical device development to other industries such as consumer electronics. This leads to increased demand and a competitive advantage. A deep understanding of customers' needs and wants will ultimately result in a brand that provides a strong emotional connection.
FAQ’s
-
Radiant was established in September 2022. Its founders, Chris and Justin, both product designers share a 13-year professional journey from their tenure at product development company, Juggernaut Design/Defense. Our collective design expertise and product development services span in a broad range of industries - Military/Defense, scientific and laboratory equipment, healthcare, point-of-sale, and consumer products. Enough about us. Let's talk about you and your project venture!
-
We can help support many phases of product development, from initial strategy through to manufacturing handoff. Or we can support you where you need us most, focusing on smaller, select phases of engagement. In most scenarios, our minimum engagement typically starts at $10,000. Let's discuss your project or idea so that we can provide a more accurate cost proposal.
-
When designing medical devices, industrial design plays a paramount role in shaping the overall success and appeal of consumer products. It extends far beyond mere aesthetics but contributes to improving functionality through human factors, leading to increasing user adoption. Triggering a positive experience through user interaction and product safety. We don't see why similar attributes can't be carried across multiple industries such as law enforcement, point of sale, biosciences, where or devices detecting or saving lives. All tools should enforce some level of confidence.
-
With so much hype recently about AI, it's important to remember AI is just a tool. It can be an excellent tool for specific tasks but not so great for others. We will utilize AI in our process in a supporting role. We contend that AI lacks certain aspects of humanity, particularly empathy, which we consider essential for a user-centered approach to problem-solving and design.
-
Yes. We understand the hurdles and complexities that come with launching a new startup or building a brand — it's no small feat. Recognizing the various challenges for small business clients, we can discuss different pricing models, or we, as partners, are prepared to contribute our design services as a form of capital tailored to the specific needs of the venture. Our creative and technical skills can guide you through the entire design process, supporting your business model and preparing you with a market-ready product. Our involvement just doesn't stop there, we provide ongoing support to evolve your product or brand. Overseeing quality control, continued user testing efforts, and consistently evaluating cost-effective updates as necessary. An application of good design principles doesn't solve new business challenges. We integrate project management, market research, and business strategy to ensure not only the successful execution of projects but also strategic alignment with market trends and long-term business goals. human factors
-
This varies. Our standard payment terms are net 15 days. We offer flexibility to our long-standing clients and established relationships, allowing for a net payment period of 30 days or more.
-
Whether starting on the journey with a single product offering or managing a multigenerational brand, a product company must effectively communicate its identity and recognition while establishing an underlying trust within its customer base. We firmly believe that without a clearly defined identity, the lack of consistency and a competitive edge can pose significant challenges for any company seeking success.
-
No. If you are a startup with a medical device idea, we can guide you to help you understand the challenging involvement of bringing a medical device to market. We can work with you to source third-party agencies to assist in the FDA approvals e.g., premarket approvals (PMA), 510(k). Like most NPD ventures, especially innovative medical devices, will require extensive support and funding for clinical trials and testing. Contact us to see if were a suitable fit for collaboration with your team and other medical device developers you may already be working with.